Performance
2023 – 2024 Annual Report
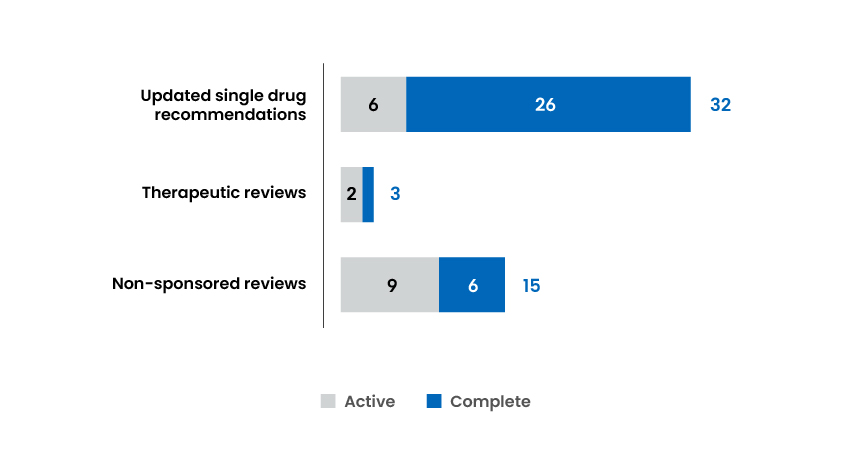
Reports and Recommendations Published

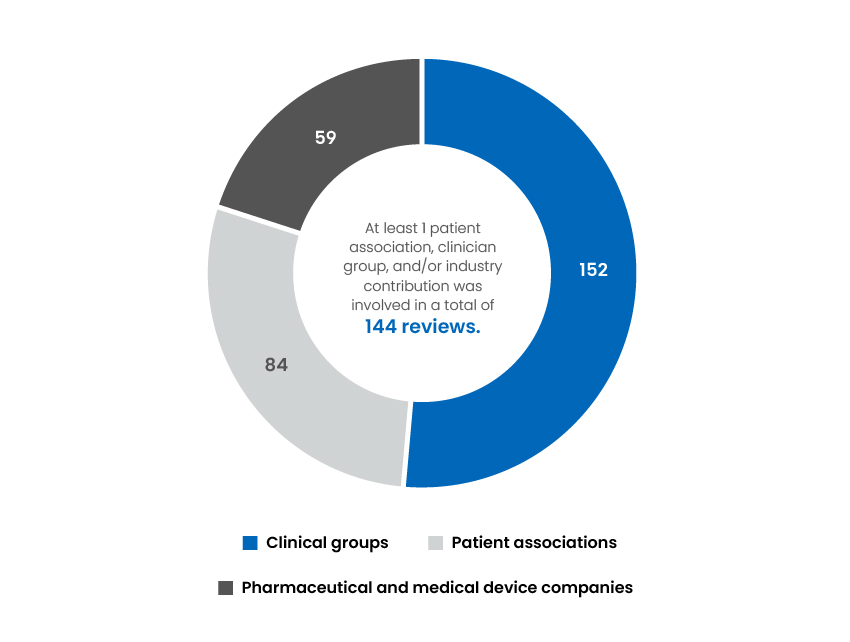
Associations That Contributed to Our Reviews
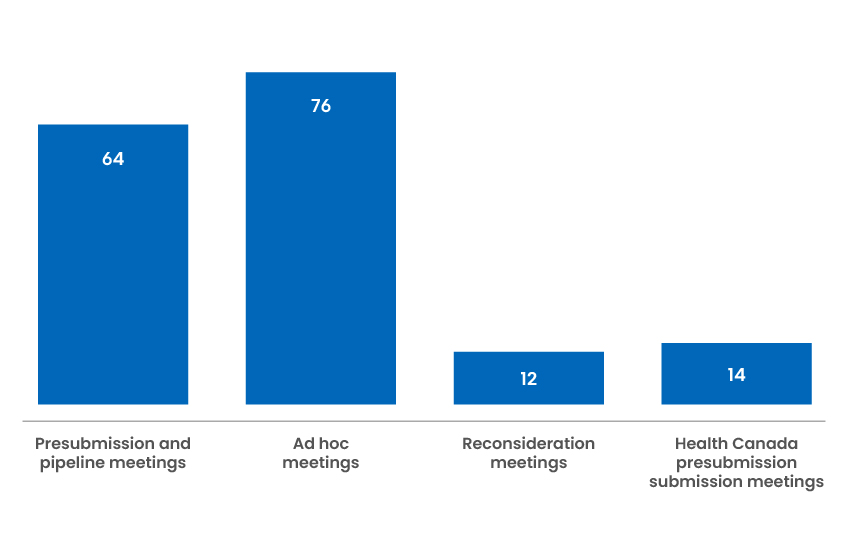
166 Industry Meetings
We offer presubmission and pipeline meetings to industry to help facilitate the preparation and filing of applications. In addition, we have been examining opportunities to improve engagement with industry sponsors, and have been able to accommodate additional meetings on an ad hoc basis to resolve potential issues in a timely manner.
Pre–Notice of Compliance Submissions and Aligned Review Participation
Formulary Management Expert Committee Activity
The Formulary Management Expert Committee (FMEC) is an interim committee that provides recommendations for the organization’s nonsponsored single drug reviews, streamlined drug class reviews, and therapeutic reviews, as requested by federal, provincial, and territorial governments; drug plans; and cancer agencies.
Symposium 2023
Symposium 2023 was held from May 16, 2023, to May 18, 2023, and saw the highest attendance of any Symposium (with registration fees) to date.
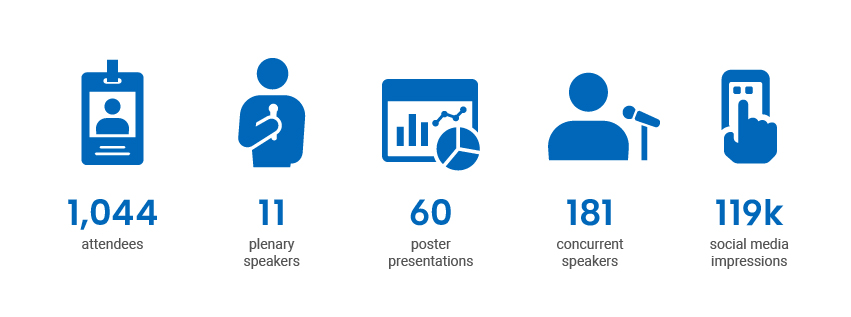
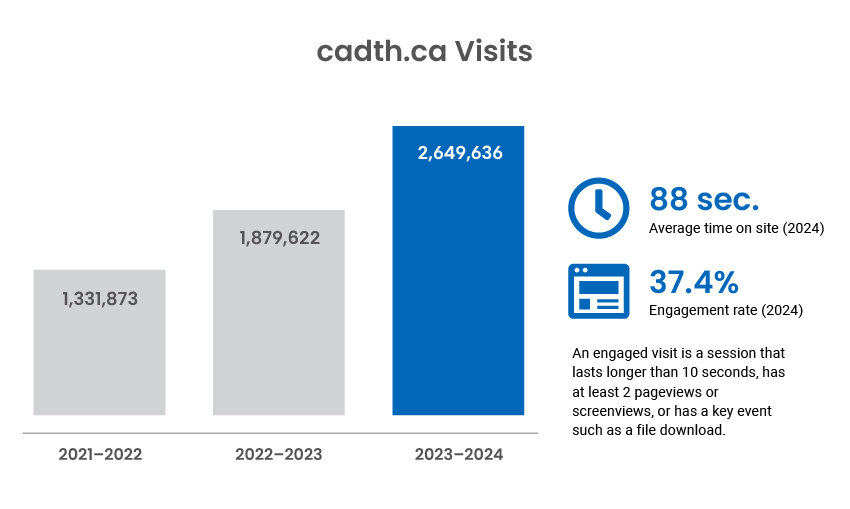
Digital Channel Activity
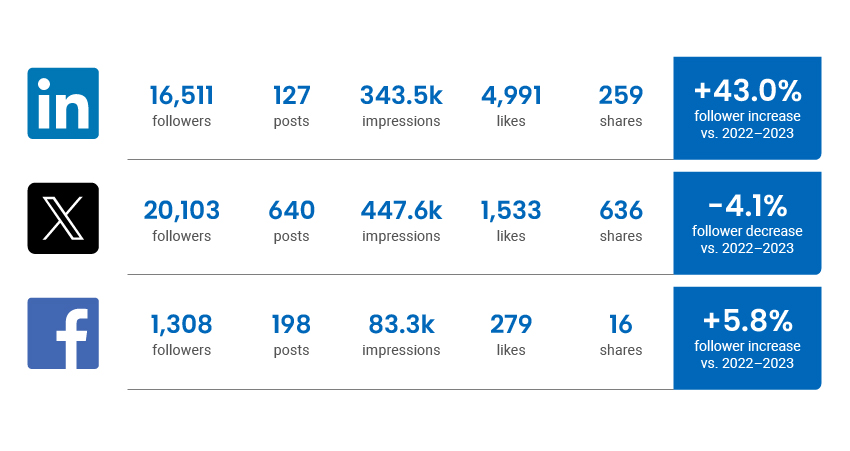
Traffic statistics and activity for cadth.ca and our official social channels.
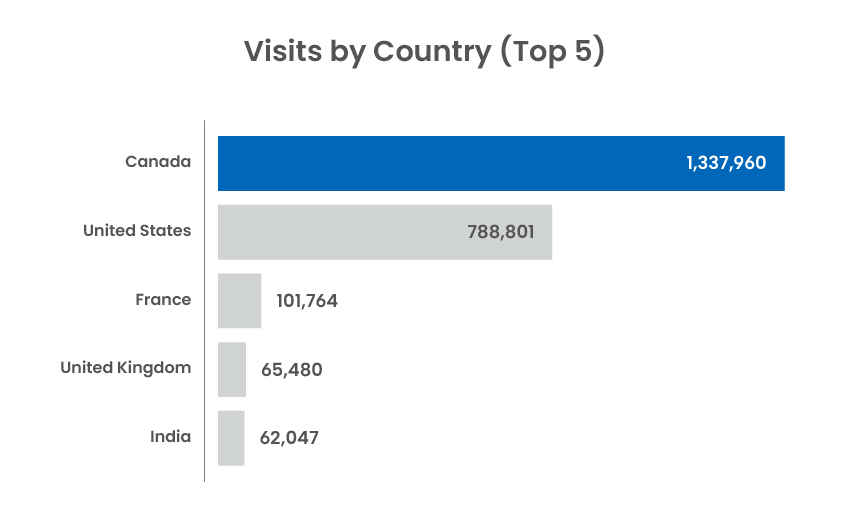
Social Media Channel Activity