Last Updated : June 5, 2023
The following frequently asked questions provide information about upcoming process improvements, as well as adjustments to our fee structures.
1. When will we communicate additional details regarding the lean review of the reimbursement review process?
Answer: We will provide additional details regarding the process improvements and enhancements to the drug review process, including the lean review, by the end of summer 2023. These will be communicated through our Weekly Summary newsletter. Subscribe to receive these updates by email.
2. When will we learn more about the other process improvement changes?
Answer: Some changes will happen immediately, including presubmission pipeline meetings, which will launch June 8, 2023. We are currently working through our plans for the other improvements, and will reach out to stakeholders shortly to engage further. Regular updates on progress will be communicated within the Pharmaceutical Review Updates (PRUs).
3. What are application fees for Drug Reimbursement Reviews?
Answer: When drug companies file a submission with us for a Drug Reimbursement Review, they pay an application fee. Application fees vary, depending on the complexity of the review. Revenues from these application fees help to offset the costs associated with the growing volume and complexity of drugs that we review annually, and ensure that we can adhere to predictable timelines for our review process. Application fees supplement funding for the drug Reimbursement Reviews from federal, provincial, and territorial governments. Our fee schedule and application types are described in Fee Schedule for Pharmaceutical Reviews.
4. What are the application fees associated with a drug Reimbursement Review in the new Fee Schedule?
Answer: Table 1 lists the application fees that will be charged on and after July 17, 2023. The application fee schedule provides broad guidance. We reserves the right to make case-by-case determinations as to the applicable fee schedule.
Table 1: Summary of Current and New Fees
| Schedule | Application type | Current fee | New fee |
|---|---|---|---|
| Applications received on or after July 17, 2023 | |||
| A | Application reviewed through the standard review process | $75,900 | $98,670 |
| C | Application reviewed through the tailored review process | $37,940 | $49,320 |
| D | Request for reconsideration of a draft recommendation | $7,380 | $9,590 |
| E | Application reviewed through the complex process | $112,400 | $146,120 |
| F | Application reviewed through the process for drugs with expanded health system implications | $132,650 | $172,450 |
5. Why are we increasing application fees above the annual CPI (Consumer Price Index) adjustment?
Answer: Our application fees were introduced in 2014 and remained unchanged until 2018, when annual adjustments based on the CPI were introduced. Those adjustments have not been sufficient to allow us to effectively manage the increasing volume and complexity of applications.
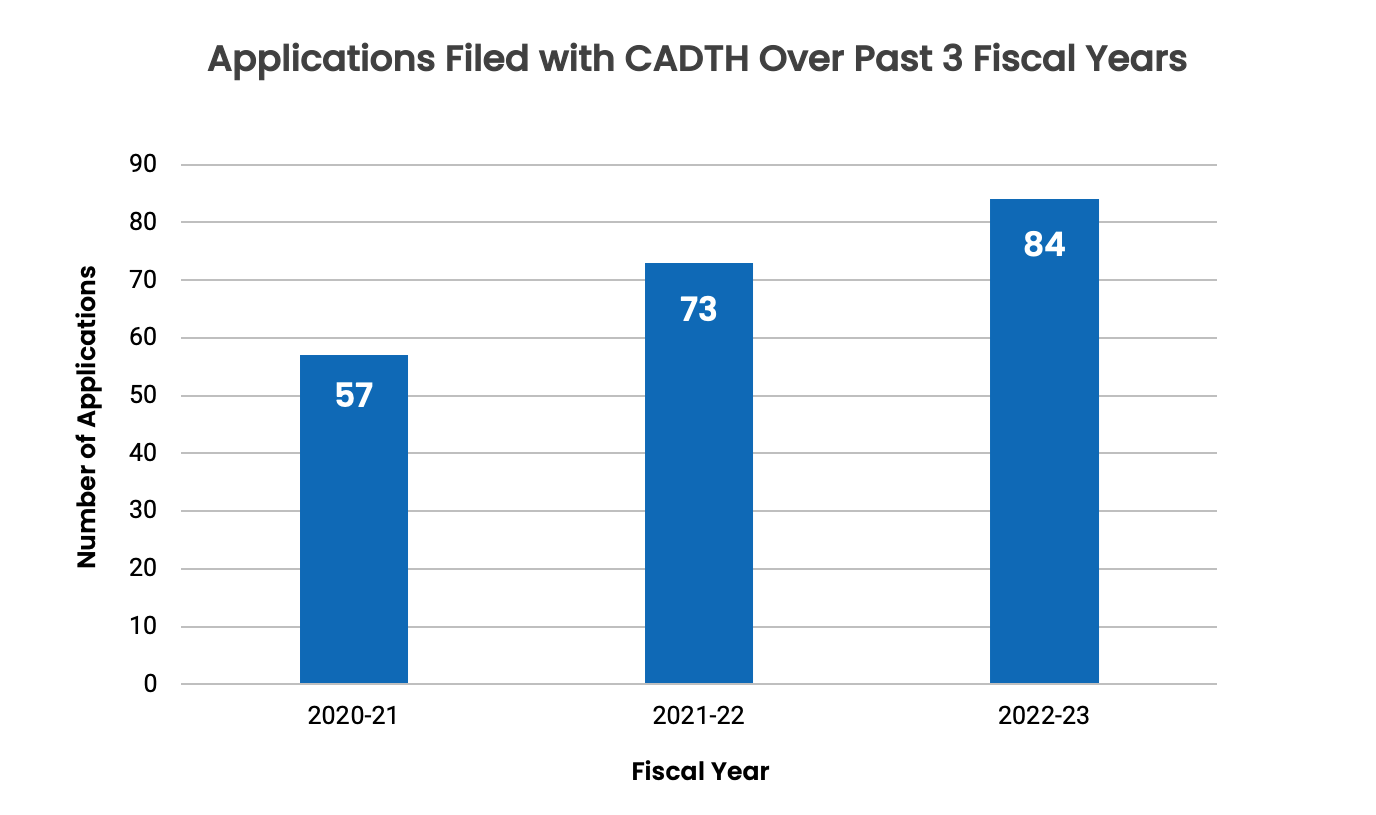
Figure 1 shows the increase in the number of drug submissions filed with us over the past 3 years, largely driven by an increase in the number of oncology submissions. Since the 2020–2021 fiscal year, the number of applications has grown by 48% (from 57 submissions to 84 submissions).
Figure 1: Applications Filed With Us Over the Past 3 Fiscal Years
In addition to growing volumes, complexity is increasing in key areas:
- Earlier regulatory approvals, in some cases, with less mature data and greater uncertainty in the evidence base
- More complex clinical data that was less common when fees were introduced
- New economic evaluations, including the appraisal of budget impact analyses, which offers substantial value and minimizes duplication by payers and the pan-Canadian Pharmaceutical Alliance (pCPA)
- Increased stakeholder engagement to seek input from patient groups and clinical societies
- More downstream implementation considerations that we must address to better support payer decision-making and pCPA price negotiations.
Within this challenging environment, we have continually expanded the scope and output of our Reimbursement Review program in response to evolving needs. Examples include a new process for plasma products, implementation advice panels, provisional funding algorithms for oncology drugs, an aligned review process with Health Canada, and process improvements to more effectively review cell and gene therapies as well as drugs for rare diseases. We have strictly adhered to performance metrics (including throughout the pandemic) that see us deliver Reimbursement Recommendations within 180 calendar days from the date of submission.
While our jurisdictional contributors increased their funding for Reimbursement Reviews last year ($470,000 annually and a 1-time injection of $410,000 in the 2021–2022 fiscal year), the program remained with a substantial deficit in 2022–2023. The increase in fees is required to ensure the sustainability and reliability of the Reimbursement Review process, address a structural deficit in the program and the unintended impacts on the nonpharmaceutical parts of the organization, and create the capacity for a modern, responsive pharmaceutical review program.
We have worked diligently with our industry and government partners to consistently meet our commitments and provide much-needed predictability in the face of change. However, it is now the time to strengthen our funding and capacity to continue to meet our obligations as Canada’s drug and health technology agency.
6. When does the new fee schedule for Reimbursement Reviews become effective?
Answer: The revised fee schedule is effective for all applications filed on or after July 17, 2023.
7. What application types are subject to the revised fee schedule?
Answer: The revised fee schedule will be applied to all applications received on or after July 17, 2023, irrespective of the application type or applicable fee schedule.
8. Can a sponsor file an application prior to July 17, 2023, if they have not provided at least 30 business days’ advance notification?
Answer: No, the advance notification procedures must be completed in accordance with section 4.2 of Procedures for Reimbursement Reviews. All sponsors must provide at least 30 business days’ advance notification prior to filing the application.
9. Will the revised fee schedule apply if a sponsor has provided at least 30 business days’ advance notification with the intent to file their application prior to July 17, 2023, but subsequently encounters an unexpected delay and must file the application after July 17, 2023?
Answer: We appreciate that sponsors may encounter unexpected delays in the days leading up to the target application date. Sponsors who have provided sufficient advance notification and were targeting a submission date prior to July 17, 2023, will be invoiced under our previous fee schedule.
10. If a sponsor has concerns about payment for the increased fees, can they contact us to discuss options for payment?
Answer: Sponsors should contact us ([email protected]) with the details of their questions and a response will be provided in a timely manner.
11. When is the revised Schedule D fee applicable for sponsors who are filing a request for reconsideration?
Answer: The revised Schedule D fee is applicable for all industry-filed requests for reconsideration filed for draft recommendations issued from the June 2023 expert committee meetings, for both cancer and noncancer drugs.
12. How do our application fees compare to other jurisdictions?
Answer: The pricing for our application fees for industry is competitive with many global HTA bodies, and in some instances fees may be lower.
13. Will the 60/40 contribution percentage between government funders and industry be maintained?
Answer: With these increases, our contribution model will move toward a more equitable (50/50) split between government and industry funders to reflect the shared value in this program.
14. How are fees for the Scientific Advice service determined?
Answer: We provide high-quality, thoughtfully considered Scientific Advice that is informed, specific, timely, and fit for purpose. Early engagement provides an opportunity for pharmaceutical companies to gain a better understanding of our perspective on the proposed clinical development plans, including real-world evidence generation plans and economic modelling plans.
The fee for this service is determined on a cost-recovery basis after a request is submitted, based on the project scope. For further details, please refer to the Standard Scientific Advice Process. Note that the Scientific Advice service is an optional offering that is separate from the Drug Reimbursement Review process.
Last Updated : June 5, 2023


