By the Numbers
Our performance
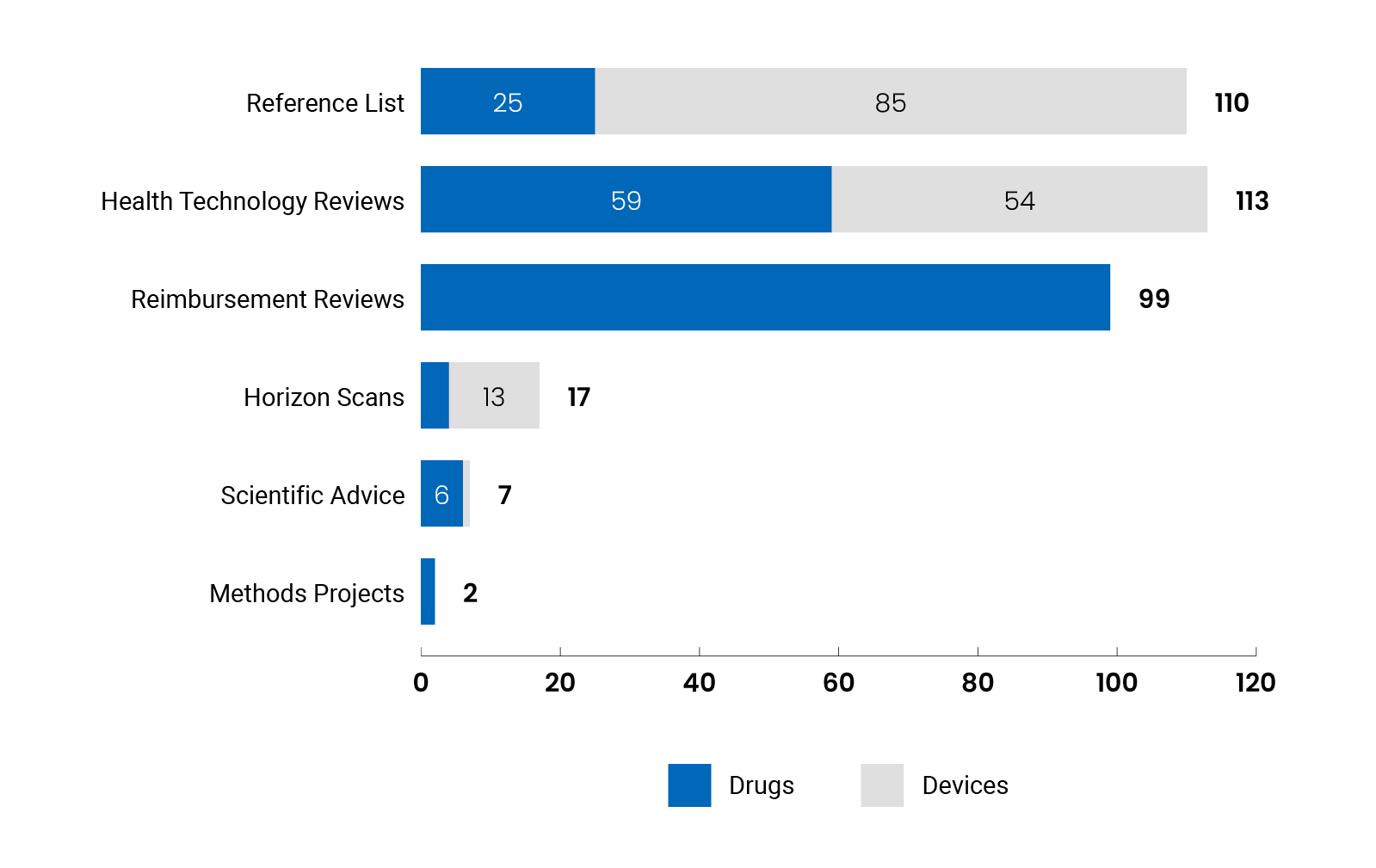
Reports and Recommendations Published by us
Implementation Tools Developed by us
Implementation Tools include supports such as Evidence Bundles, In Briefs/Summary Tools and Responding to Informal Customer Asks.


Knowledge Events

In 2022-2023 we hosted several knowledge events to advance the uptake and impact of evidence across Canada. We paused our annual Symposium conference in 2022 in order to return to a hybrid event in 2023.
Overview of Reimbursement Review Program
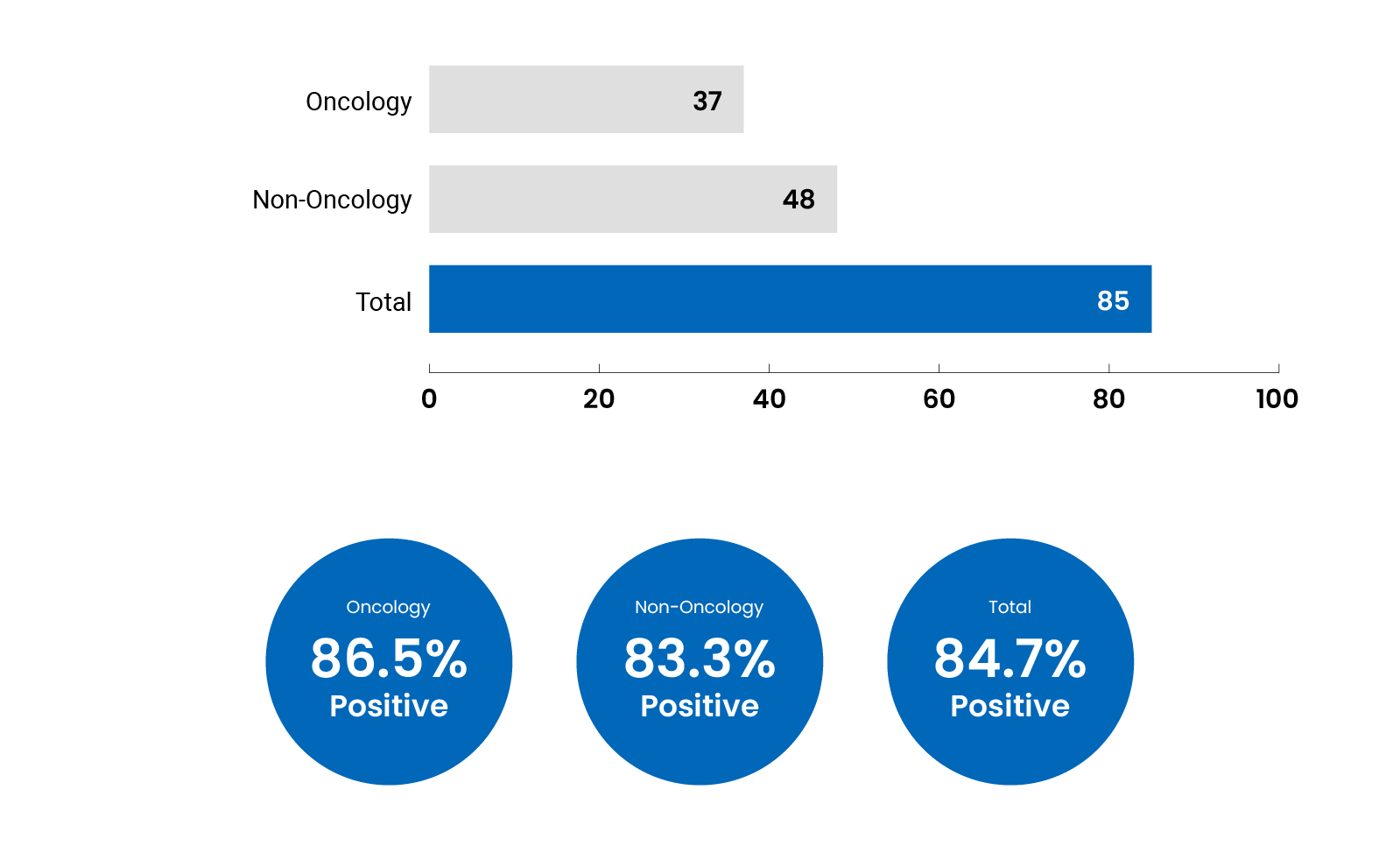
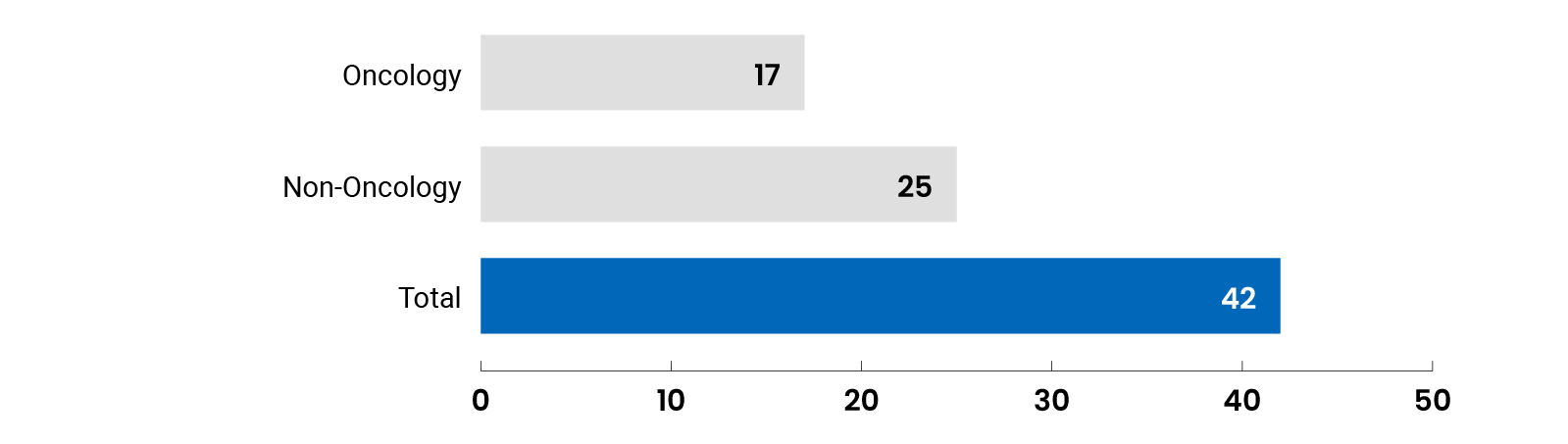
Summary of Reimbursement Recommendations in 2022-2023
Pre-NOC Drug Reimbursement Reviews
A sponsor can submit a drug to us for review up to 180 days before it has been formally approved by Health Canada and received its Notice of Compliance (NOC). Pre-NOC reviews can help decrease the time between regulatory approval and formulary listing.
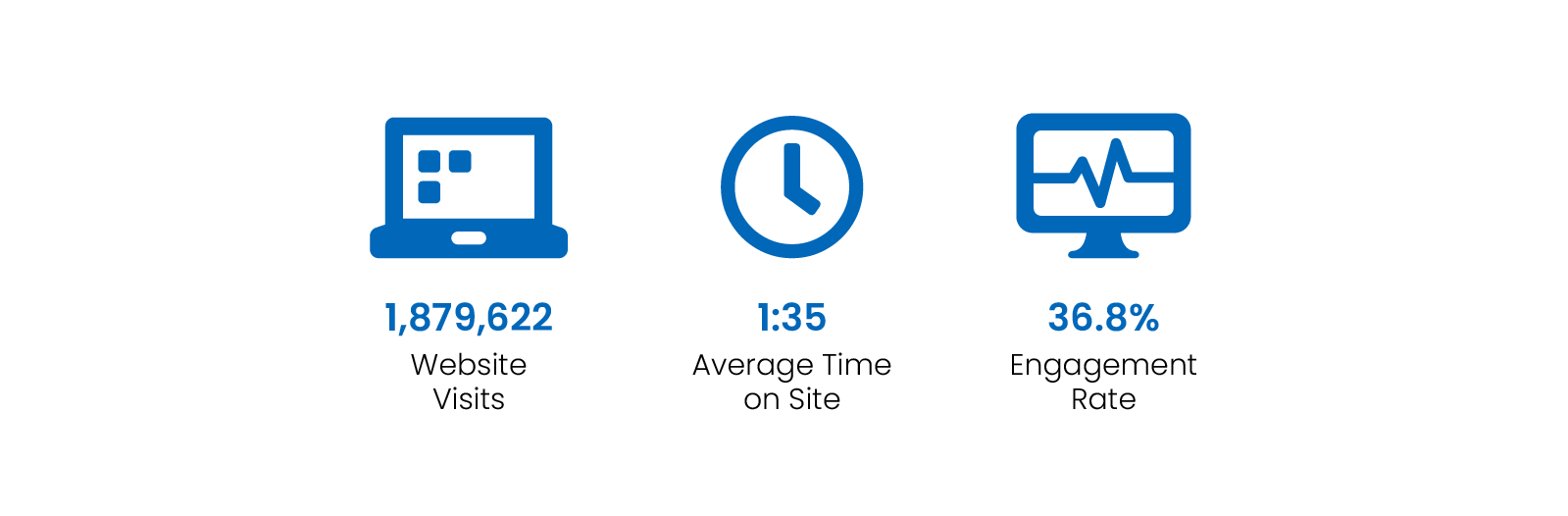
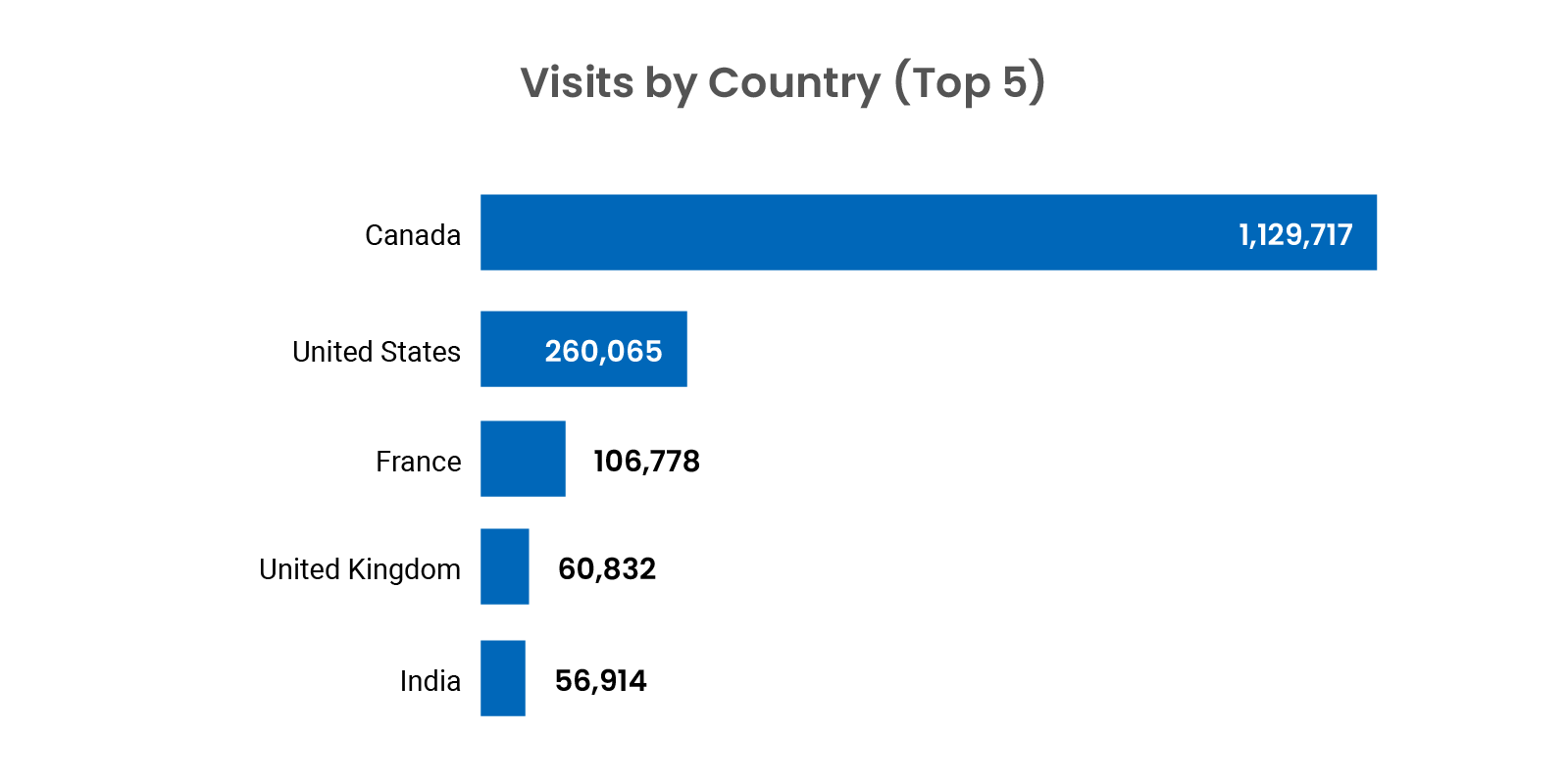
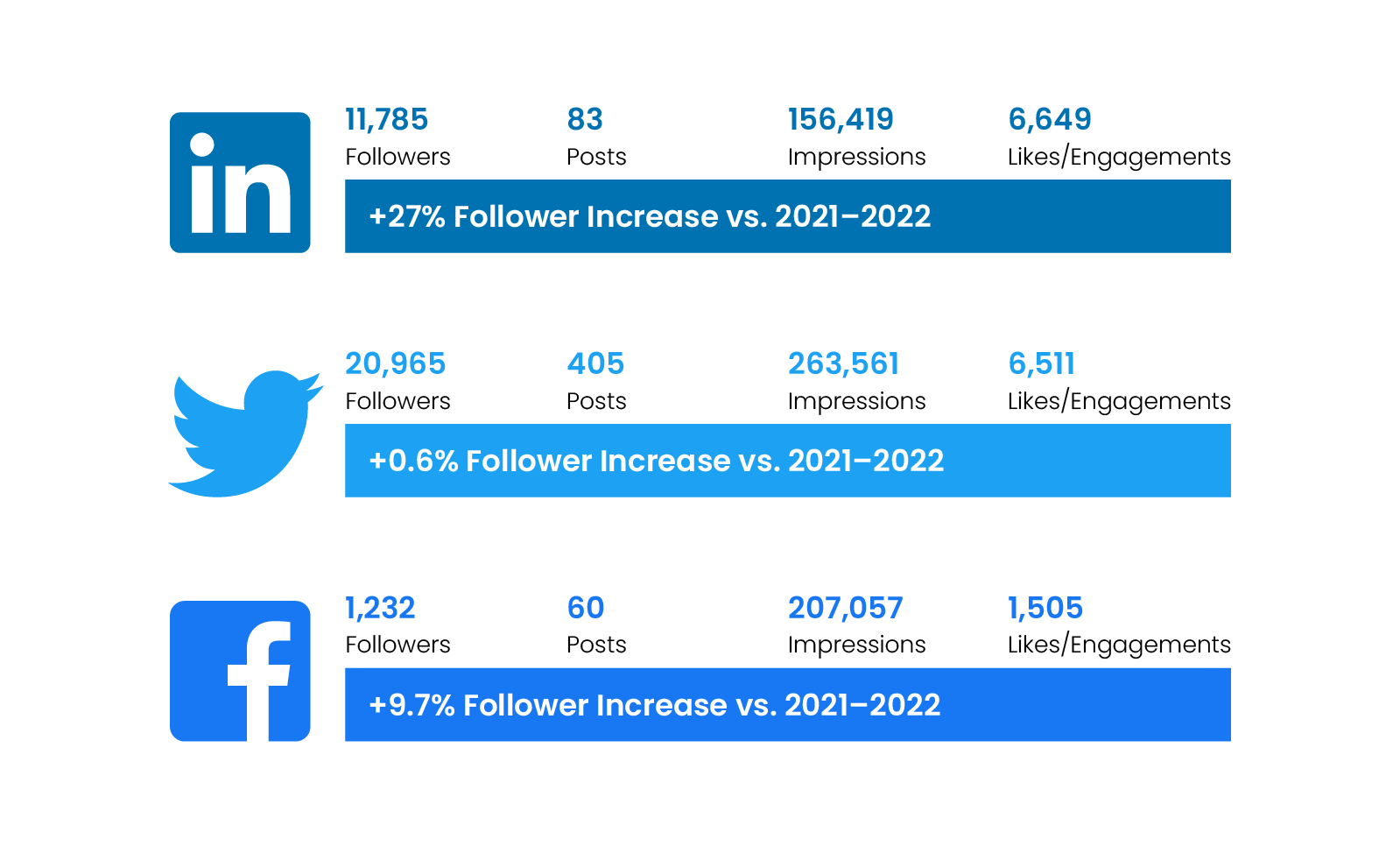
Digital Channel Activity
Traffic statistics and activity for cda-amc.ca